THE TIBSOVO + AZACITIDINE CLINICAL STUDY
TIBSOVO was studied in the following patients:
- 146 adult patients with newly diagnosed acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation who were 75 years or older or had health problems that prevented the use of certain chemotherapy treatments
All patients received azacitidine for 7 days every 4 weeks, along with either:
- 500 mg of TIBSOVO taken orally once a day, or
- A daily oral placebo
Patients receiving TIBSOVO + azacitidine or placebo + azacitidine were treated for at least 6 four-week treatment cycles unless:
- Their AML progressed, or
- They had certain side effects so their doctor decided to stop treatment, or
- They received a stem cell transplant
TIBSOVO may cause serious side effects, including changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening.
BENEFITS SEEN WITH TIBSOVO IN COMBINATION WITH AZACITIDINE IN NEWLY DIAGNOSED AML
More people with newly diagnosed AML who received TIBSOVO + azacitidine were able to live longer and achieve remission
Median overall survival (OS)a


TIBSOVO + azacitidine continues to help more people live longer than those treated with azacitidine aloned
aMedian overall survival is defined as the length of time from the start of treatment in a clinical trial to the time when half of the patients are still alive.
bA median follow-up period of 15.1 months in people treated with TIBSOVO + azacitidine for the initial OS analysis.
cA longer median follow-up period of 28.6 months in people treated with TIBSOVO + azacitidine for the long-term OS analysis.
dThe median OS for the placebo + azacitidine treatment group was 7.9 months for the initial and long-term OS analysis.
In the clinical study, 51% of people (37 out of 72) on TIBSOVO + azacitidine achieved complete remission (CR) or complete remission with partial hematologic recovery (CRh) compared with 18% of people (13 out of 74) on azacitidine alone.
34 people reached CR on TIBSOVO + azacitidine:
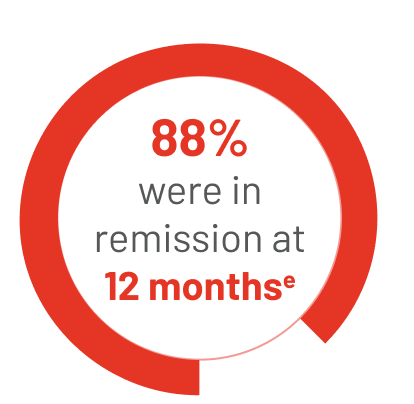
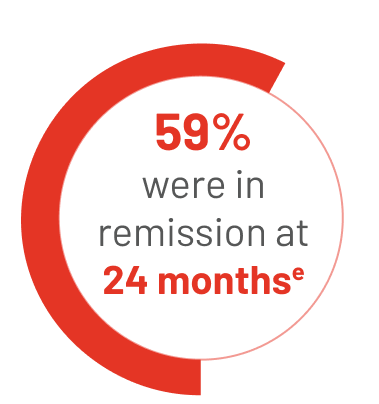
eThe median length of time that people stayed in remission could not be estimated in the study.
TIBSOVO + azacitidine also helped reduce the need for red blood cell or platelet transfusions.
Learn more about treatment benefits with the TIBSOVO AML Patient Brochure
Download nowTHE TIBSOVO CLINICAL STUDY
TIBSOVO was studied in the following patients:
- 28 adults with newly diagnosed AML who were 75 years or older or had health problems that prevented the use of certain chemotherapy treatments
- 179 adults with relapsed or refractory AML
All patients received TIBSOVO 500 mg orally to start. They were treated with TIBSOVO until:
- Their AML progressed, or
- They had certain side effects so their doctor decided to stop treatment, or
- They received a stem cell transplant
Benefits seen with TIBSOVO ALONE in newly diagnosed AML
Some people with newly diagnosed AML who received TIBSOVO were able to achieve remission.

In the TIBSOVO clinical study, 3 in 7 people (12 out of 28) achieved CR or CRh.
Of the 12 people with newly diagnosed AML who achieved CR or CRh on TIBSOVO:

fThe median length of time that people stayed in remission could not be estimated in the study.
TIBSOVO also helped reduce the need for red blood cell or platelet transfusions.
41% of people (7 out of 17) became transfusion free.
55% of people (6 out of 11) who were transfusion free when they started TIBSOVO remained so for a period of at least 8 weeks.
Benefits seen with TIBSOVO in relapsed or refractory AML
Some people with relapsed or refractory AML who received TIBSOVO were able to achieve remission.

In the TIBSOVO clinical study, about 1 in 3 people (57 out of 174) achieved CR or CRh.
Of the 57 people with relapsed or refractory AML who achieved CR or CRh:



TIBSOVO also helped reduce the need for red blood cell or platelet transfusions.
37% of people (41 out of 110) became transfusion free.
59% of people (38 out of 64) who were transfusion free when they started TIBSOVO remained so for a period of at least 8 weeks.
What is TIBSOVO?
TIBSOVO is a prescription medicine used to treat adults with an isocitrate dehydrogenase-1 (IDH1) mutation with acute myeloid leukemia (AML):
- with newly diagnosed AML treated in combination with azacitidine or alone who are 75 years or older or who have health problems that prevent the use of certain chemotherapy treatments
- when the disease has come back or has not improved after previous treatment(s)
Your healthcare provider will perform a test to make sure that TIBSOVO is right for you. It is not known if TIBSOVO is safe and effective in children.
IMPORTANT SAFETY INFORMATION
TIBSOVO may cause serious side effects, including:
- Differentiation syndrome. Differentiation syndrome is a serious condition that affects your blood cells and may be life-threatening or lead to death. Differentiation syndrome in adults with AML has happened as early as 1 day and up to 3 months after starting TIBSOVO. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with TIBSOVO:
- fever
- cough
- trouble breathing
- rash
- decreased urination
- dizziness or lightheadedness
- rapid weight gain
- swelling of your arms and legs
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) before and during treatment with TIBSOVO. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint
- Guillain-Barré Syndrome has happened in people treated with TIBSOVO. Your healthcare provider will monitor you for nervous system problems and will permanently stop your treatment with TIBSOVO if you develop Guillain-Barré syndrome. Tell your healthcare provider right away if you develop any signs or symptoms of Guillain-Barré syndrome, including:
- weakness or tingling feeling in your legs, arms, or upper body
- numbness and pain on one side or both sides of your body
- any changes in your ability to see, touch, hear, or taste
- burning or prickling sensation
- difficulty breathing
The most common side effects of TIBSOVO when used in combination with azacitidine or alone in adults with AML include:
- changes in certain blood cell counts
- diarrhea
- increased blood sugar
- fatigue
- changes in certain liver function tests
- swelling of arms or legs
- decreased levels of electrolytes in the blood
- nausea
- vomiting
- decreased appetite
- joint pain
- shortness of breath
- uric acid increased
- stomach (abdominal) pain
- changes in certain kidney function tests
- pain or sores in your mouth or throat
- rash
- irregular heart rhythm or heartbeat (QTc prolongation)
- differentiation syndrome
- muscle pain
Your healthcare provider will do blood tests before you start and during treatment with TIBSOVO. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with TIBSOVO if you develop certain side effects.
TIBSOVO may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of TIBSOVO. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before taking TIBSOVO, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndrome
- have problems with abnormal electrolytes, such as sodium, potassium, calcium, or magnesium levels
- have nervous system problems
- have problems with your kidneys or are on dialysis
- have any liver disorders, including cirrhosis
- are pregnant or plan to become pregnant. TIBSOVO can cause harm to your unborn baby. You should avoid becoming pregnant during treatment with TIBSOVO. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with TIBSOVO
- are breastfeeding or plan to breastfeed. It is not known if TIBSOVO passes into your breast milk. Do not breastfeed during your treatment with TIBSOVO and for 1 month after your last dose of TIBSOVO
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take hormonal contraceptives. TIBSOVO may affect how hormonal contraceptives work and may cause them to not work as well.
Please see Full Prescribing Information, including BOXED WARNING for AML and MDS patients and Medication Guide.
What is TIBSOVO?
TIBSOVO is a prescription medicine used to treat adults with an isocitrate dehydrogenase-1 (IDH1) mutation with acute myeloid leukemia (AML):
- with newly diagnosed AML treated in combination with azacitidine or alone who are 75 years or older or who have health problems that prevent the use of certain chemotherapy treatments
- when the disease has come back or has not improved after previous treatment(s)
• myelodysplastic syndromes (MDS):
- when the disease has come back or has not improved after previous treatment(s)
IMPORTANT SAFETY INFORMATION
TIBSOVO may cause serious side effects, including:
- Differentiation syndrome. Differentiation syndrome is a serious condition that affects your blood cells and may be life-threatening or lead to death. Differentiation syndrome in adults with AML has happened as early as 1 day and up to 3 months after starting TIBSOVO. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with TIBSOVO:


